According to conventional wisdom, a SARS-CoV-2 variant becomes more infectious when it mutates to better bind and invade host cells. But a new study on omicron, which carries quadruple the mutations of prior variants, challenges that view.
Boston Children’s Hospital virologist Bing Chen, with the help of scientists at the Institute for Protein Innovation (IPI), showed that, compared to delta, omicron is weaker.
When the receptors the virus uses to invade cells were scarce, omicron couldn’t invade them at all. Its spike proteins are also more unstable, dampening their infection potential.
At the same time, omicron’s ensemble of mutations renders the variant remarkably deft at evading our immune defenses. It appears the virus may have swapped potency for secrecy.
“The fact is that omicron took over very rapidly, that implied a better virus,” Chen said. “But from the spike protein point of view, I would say this is really sort of a compromised version.”
The study suggests that there may be a limit to how deadly future variants can be — unless one evolves a radical combination of mutations that dramatically alter viral mechanics.
A titan among variants? It may not be so simple
The basis of all this variation is the SARS-CoV-2 spike proteins. These stud the coronavirus’ surface, giving rise to its medieval weapon-like look. Spike proteins latch onto a cellular receptor, called ACE2, tethering and pulling the virus into host cells.
Chen’s research reaffirmed that a crucial part of omicron’s spike proteins, their receptor-binding domain, has changed far less dramatically in its overall structure than other parts of the molecule targeted by neutralizing antibodies. That discovery has held since the pandemic’s start and offers a template for what scientists can expect from future variants.
Chen’s work also helped researchers make sense of contradictory findings about omicron’s behavior.
Early on, scientists from the University of Hong Kong discovered that omicron mounted infections 70 times faster than some previous variants. Those results conflicted with another preprint showing that patients infected with omicron had lower odds of requiring intensive treatment. Yet another study showed that omicron was no more likely to be spread among unvaccinated members of the same household than delta, indicating that omicron wasn’t more contagious.
If omicron was a better virus, why, in many cases, did it seem to cause less severe disease? And if heightened transmissibility might not be to blame for its spread, what was?
To find out, Chen first tested how well and quickly omicron bound to human cells. It quickly fell behind, fusing to cells slightly slower and more weakly than other variants. In fact, omicron required roughly 10 times the level of ACE2 receptors to successfully bind to cells at the same rate as previous variants.
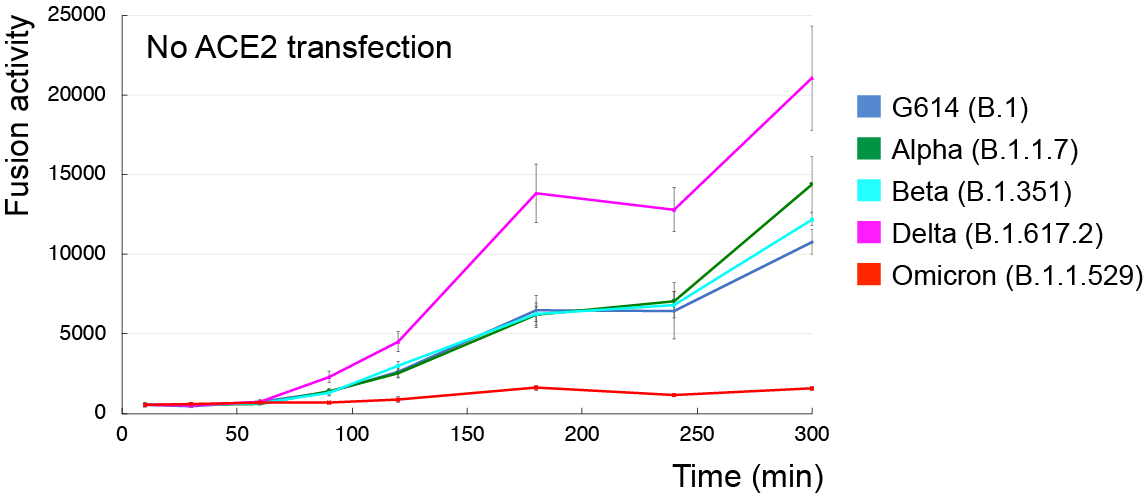
But its lackluster pace was bolstered by its clever evasion of nearly every antibody known to stifle previous coronavirus variants.
“This is really the worst case in terms of vaccine efficacy,” Chen said.
To zero in on the molecular interactions between virus and cell, Chen turned to Haisun Zhu, a principal scientist at IPI, and Krishna Anand, a research associate. The pair used flow cytometry, which floats one cell at a time through a laser beam to reveal its physical and chemical properties, to map how omicron’s spike proteins bound to receptors and antibodies.
The researchers found that, once omicron was able to bind, it did so more strongly than some previous variants, including early variant G614. The findings mirrored those of another study led by researchers at the University of Washington and Vir Biotechnology, leaving Chen’s team with a new puzzle.
If omicron’s spike bound more tightly to ACE2 receptors, why did the variant need so many receptors to get into cells? The answer lay in its structure, which Chen began to unravel.
All SARS-CoV-2 spike proteins have two key regions. The receptor-binding domain (RBD) orchestrates how SARS-CoV-2 enters cells, while the N-terminal domain (NTD) shields the RBD from antibodies that may target it.
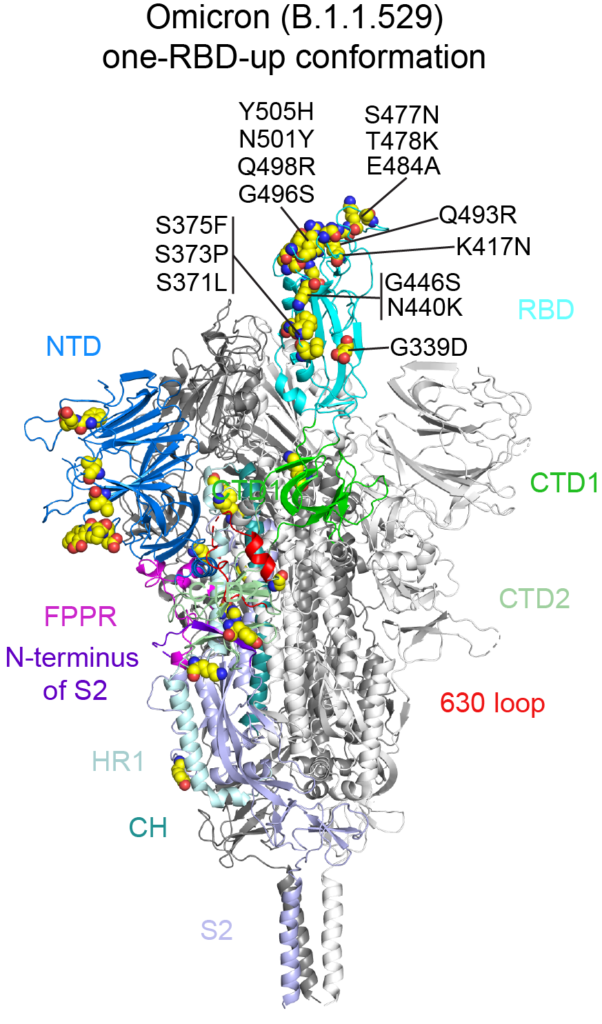
To hide from our immune systems, the RBD takes two shapes: a down confirmation, where it’s hidden by the NTD, and an up confirmation, when the RBD is exposed and ready to bind to ACE2 receptors. As a result, SARS-CoV-2 can bind to cells only when the RBD shifts upward.
Chen’s research showed that one of omicron’s many mutations stiffened a region of the spike close to the RBD. It may have inadvertently cost its spike the flexibility to shift the RBD up, forcing the RBD and the now-rigid segment to clash as the virus attempted to latch onto cells.
In other words, omicron’s mutational repertoire boosted its ability to hang onto cells — but disabled its ability to lock on in the first place.
“It doesn’t matter how high affinity the RBD has for ACE2. You have to move to the up confirmation in order to see ACE2, right?” he said. “That is the problem for omicron.”
It’s a nuance that, if borne out by more research, would have been missed by previous studies. That’s because Chen’s team expressed omicron’s full-length spike protein using an unmodified genetic sequence. Other teams expressed stabilized, soluble protein fragments to trap them in a defined shape.
Though those soluble fragments are faster to create, ultimately enabling faster results, they could have missed certain critical regions of the protein, confounding the results in a way the full-length form Chen’s group made wouldn’t.
Wearing a new disguise, omicron still poses a fierce threat
Combined, omicron’s mutations did something else: They enabled the variant to evade nearly every antibody that had neutralized previous coronavirus variants.
Again using flow cytometry, IPI’s Zhu and Anand tested three antibodies that bound to the RBD, three that bound to the NTD and one that bound to the stalk of omicron’s spike. Only one, which targeted part of the RBD, bound even slightly.
The new findings back previous research by Chen, Zhu and Anand that suggests the RBD is the most reliable region of the virus to target with vaccines. Out of roughly 60 mutations across the entire virus, 15 were to the RBD, keeping its overall shape constant to preserve its critical function: engaging host cells.
That helps predict how future variants might evolve. Charged with shielding the RBD from existing antibodies, the NTD will likely continue to shapeshift. Meanwhile, the RBD will likely hold relatively steady. While the virus could overhaul the entire spike protein, it would take many mutations, in a dramatic combination, to alter the RBD’s mechanics. Still, Chen cautions against presuming that SARS-CoV-2 variants will become progressively milder.
“It might be still a bit early to tell,” he said.
More data has to emerge before scientists can tell whether omicron’s ability to evade our antibodies is entirely to blame for omicron’s rapid ascent and obliteration of previous variants.
“For me at the moment, it’s still sort of puzzling why omicron is taking over so fast. I don’t think anyone has sort of a really satisfactory answer for that yet,” he said.
Writer: Halle Marchese, halle.marchese@proteininnonvation.org
Source: Bing Chen, media.relations@childrens.harvard.edu