If you were diagnosed with aggressive neuroblastoma, you’d most likely be an infant carrying a genetic change that sprang up in your nerve cells before you were born. That change might lie in a gene called MYCN, which oversees the DNA-to-RNA transcription of specific targets.
If you are unlucky, your tender age, cancer stage and location would land you in a high-risk group, forecasting a five-year, 50% survival rate. MYCN, when dysregulated, lashes out, fueling tumor growth and progression that is notoriously difficult to treat.
Yet hope — in the form of at least understanding this renegade oncogene — comes from a recent study led by Hui Feng, an associate professor of pharmacology, physiology and biophysics at the Boston University Chobanian & Avedisian School of Medicine. Published in Science Advances, the work employs protein tools — including an Institute for Protein Innovation (IPI) recombinant antibody — to implicate chemokine-like factor (CKLF), a cytokine trigger for immune responses, in the early stages of neuroblastoma growth.
Clinically, “this study takes on a complex protein mechanism that has important implications for cancer treatment,” says Rob Meijers, senior director of the Antibody Platform at IPI and a co-author on the study. “We’re pleased to have been a part of it.”
Finding the mechanisms
To conduct the study, the Feng Lab examined the mechanisms of MYCN-mediated immunosuppression, analyzing human solid tumors and live tracking the tumor microenvironment in a zebrafish cancer model.
“We discovered that CKLF is highly expressed by various solid tumor cells and transcriptionally upregulated by MYCN,” the authors say.

In the early stages of MYCN-mediated cancers, the researchers found tumor cells secrete CKLF. Specifically, CKLF attracts and binds the chemokine receptor type 4 (CCR4), expressed on immune-directive CD4+ T cells.
While such T cells are invaluable immune actors — activating immune responses, including natural killer (NK) cell deployment, during injury or microbial invasion, for example — the cells can also suppress inappropriate immune activation, preventing autoimmune responses.
The Feng Lab discovered that CKLF particularly recruits a T cell subset called FOXP3+ T regulatory cells (Tregs), which suppress tumor responses and favor tumor progression. This Treg pro-tumor activity, the authors found, is at the core of MYCN-mediated tumor aggressiveness.
Depleting the number of CD4+ Treg cells would negate this immunorestrictive and pro-tumorigenic effect. However, Treg cells have proved difficult to target. Instead, the researchers focused their attention on the identification and characterization of CKLF in immunosuppression and tumor aggressiveness.
Zebrafish models
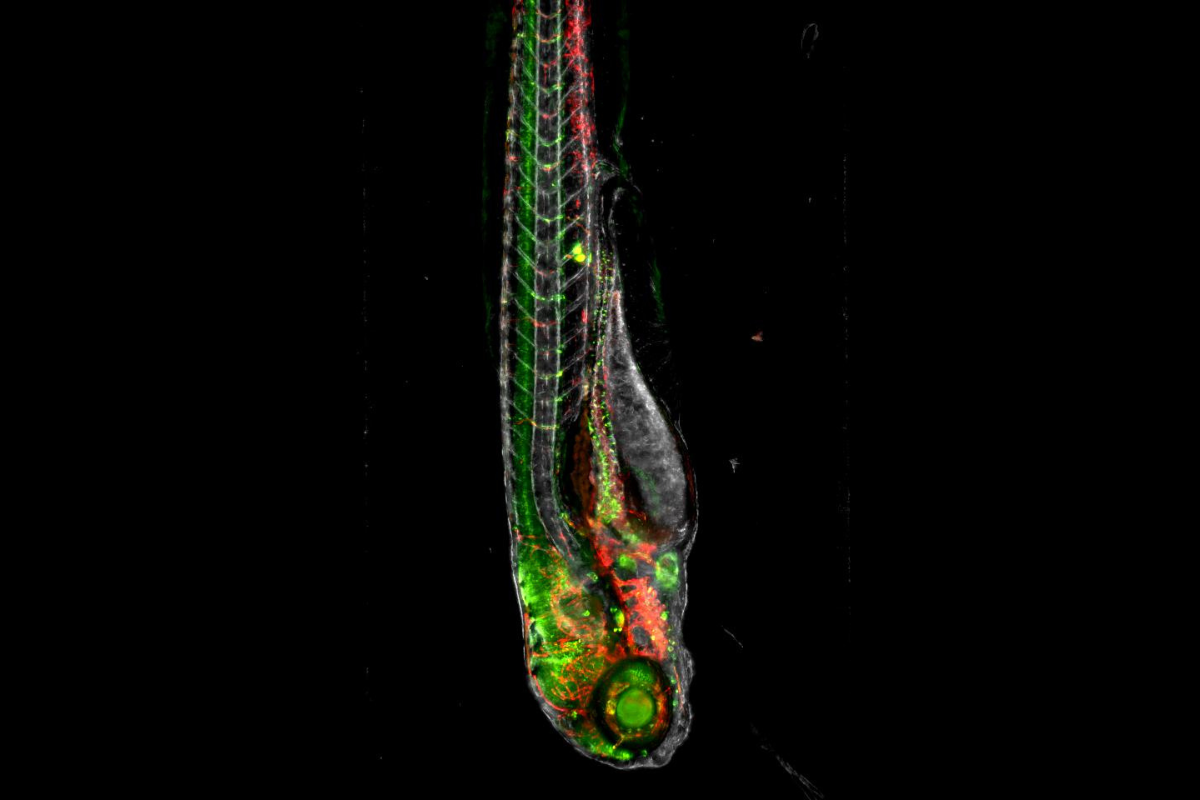
To test the role of CKLF in human disease, Feng’s group gathered a contingent of antibodies to stain tissue samples obtained from neuroblastoma patients. In addition, the researchers harnessed the genetic power of zebrafish, a model organism valuable in modeling human diseases.
For Feng, director of the Boston University Laboratory of Zebrafish Genetics and Cancer Therapeutics and a collaborator and colleague of IPI Scientific Advisory Board Member Leonard Zon, zebrafish were a natural choice. These small, striped creatures share about 70% of human genes and many of the critical pathways required for the development of the nervous system, bones and cartilage, organs and more. Zebrafish breed rapidly, allowing experiments to be repeated and genetic studies to be accelerated. Plus, zebrafish embryos are transparent, providing researchers with a way to view live embryo development and fluorescent labeling.
That transparency also offers a unique window to antibody-mediated viewing. By conjugating a tag or dye to an antibody, researchers can track cellular pathways and better understand cellular systems.
Still, it’s difficult to attain zebrafish-specific antibodies by traditional polyclonal or hybridoma methods. The process requires the production of zebrafish antigens, which is not straightforward using standard antigen production methods. And, while there is a sizable zebrafish community, the economic incentive to produce antibodies for a model system like zebrafish is limited.
“That really handicaps the field,” Meijers says. “If the zebrafish community had good antibodies, it would transform the field.”
For this reason, the Feng Lab reached out to IPI in search of antibodies targeted to zebrafish neural cell adhesion molecule 1 (NCAM1). The molecule is expressed on the surface of NK cells, with cell-destructive, or cytolytic, powers that can take out emerging tumor cells. NCAM1 can track NK cell abundance as MYCN-mediated neuroblastoma progresses.
IPI developed zNCAM1 antibodies in-house, ensuring their specificity with a series of biophysical assays, including mass spectrometry, size exclusion chromatography and surface plasmon resonance. The Feng Lab then used one anti-zNCAM1 to show that, when CKLF is increased in MYCN-driven cancers, NK cell numbers declined — therefore, affirming the crucial role of CKLF in immunosuppression and tumor aggression. This discovery suggests that therapeutic antibodies or small molecule inhibitors could interrupt CKLF’s interaction with the CCR4 receptor, quieting Treg pro-tumor activity and improving patient outcomes in neuroblastoma and other MYCN-driven cancers.
And there’s likely more discovery that an NCAM1 antibody can help tease out.
NCAM on the mind
NCAM1 functions in neurological processes, including neurogenesis, axonal branching, cell migration and synaptic plasticity. These and others neuroscientific foci are now front and center at IPI in its quest to develop antibodies for specific scientific communities in need of protein tools.
Stay tuned, as IPI launches distribution of its first antibody sets with the help of the nonprofit biorepository, Addgene.
“We’re actively working on preparing our antibodies for the Addgene catalog. And we plan to share as much experimental detail on the use of these antibodies as possible, so researchers can decide for themselves what applications are useful,” Meijers said. “It’s very exciting.”
Source: Rob Meijers, rob.meijers@proteininnovation.org
Writer: Caitlin Faulds, caitlin.faulds@proteininnovation.org
About IPI
The Institute for Protein Innovation is pioneering a new approach to scientific discovery and collaboration. As a nonprofit research institute, we provide the biomedical research community with synthetic antibodies and deep protein expertise, empowering scientists to explore fundamental biological processes and pinpoint new targets for therapeutic development. Our mission is to advance protein science to accelerate research and improve human health. For more information, visit proteininnovation.org or follow us on social media, @ipiproteins.
The zebrafish Ncam-1 antibody — IPI-zNcam-1 — used as a marker for NK cells, is available for research studies from The Institute for Protein Innovation. For more information, contact antibodies@proteininnovation.org.