At the heart of all nervous system functions are the synapses, where signals fly between neurons in swarms of molecular activity. Thomas Biederer is intent on prying open the compartments’ complex workings. And for that, he needs tools.
“The challenges of getting good tools, and the need for good tools — I experience this every day,” he said during a recent seminar hosted by the Institute for Protein Innovation (IPI). “This is really a fundamental task.”
Biederer, an associate professor of neurology at Yale University School of Medicine and senior scientist at the USDA Human Nutrition Research Center on Aging at Tufts University, speaks from deep experience. Beginning as a postdoctoral fellow in the lab of Thomas Südhof, he for almost 25 years has been tracking synapses’ molecules and mechanisms — work that relies on imaging technologies and molecules like antibodies that recognize synaptic proteins.
We sat down with Biederer to learn about the mysteries in synapse biology still waiting to be solved, and the experimental approaches he and others in the field are developing. Our interview has been edited for length and clarity.
For you, what makes synapse biology such a fertile ground for investigation?
It’s my entry point to understanding neuroscience. I can apply my interests as a biochemist to understanding the molecular composition of synapses. But I can also look at the functional relevance — how differences in synapses impact connectivity, how they alter network properties, how they contribute to cognition. That’s for me the beauty of synapse biology: You can investigate it across scales, from a cell biological to a systems neuroscience level.
Why is tracking synapses’ molecular composition so especially valuable?
Synapses are more than just little dots that connect neurons into networks. They change over time. Some are more active than others. And they are distributed in a very precise manner. Knowing their molecular makeup will allow us to understand how they encode their properties.
There seem to be infinite questions about the molecular-level workings of synapses. At a high level, what do researchers want to learn?
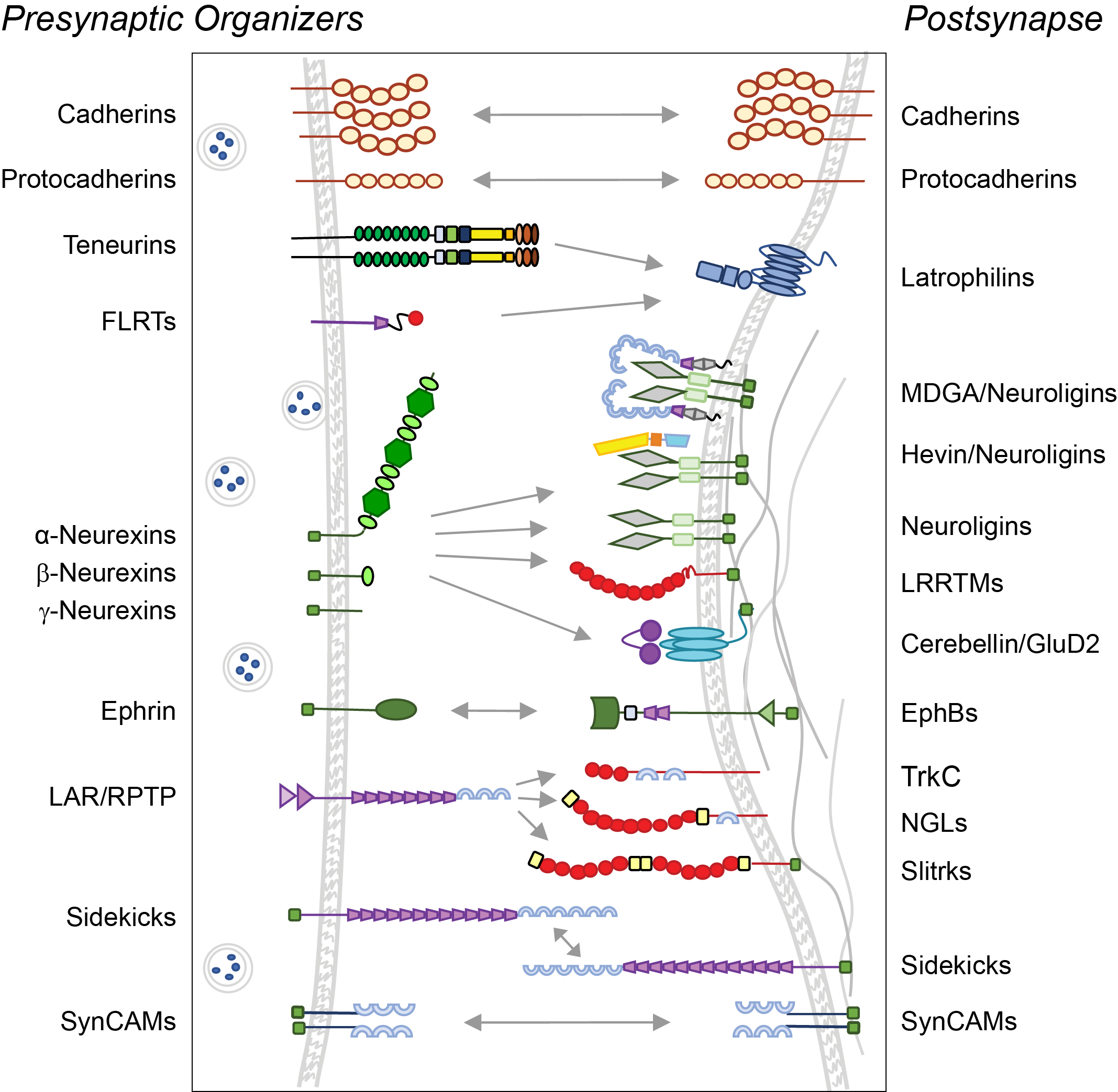
One way to think about that is to look at our diagrams depicting synaptic molecules and their interactions. Think of the facts that we assume when we draw these diagrams but that we don’t actually know.
We don’t know which molecules are at a given synapse at the same time. We have limited understanding of how the molecular composition changes as synapses develop or as they change strength. Also, we don’t know the nanoscale locations of proteins within a synapse or their dynamic properties, which are really fundamental to how a synapse functions.
We don’t know which molecules make synapses different; we also don’t know the copy numbers of these proteins. It could very well be that a synapse that has five copies of a given protein is very different from a synapse that has 50 copies. So it’s maybe not just whether a synapse has something or not that matters, but also how much of it, and where it is.
How can antibodies be most helpful in addressing those questions?
On the one hand, we need to map the diversity of synapses in healthy brain development and function. We look at markers that are shared across the biggest subtypes of synapses — but we may not be able to pick up changes between very distinct populations. We need antibodies to track those changes.
This is also really important for looking at synapse vulnerability in disease. For example, in neurodegeneration, if you want to look for interventions that help to stabilize synapses — my lab is doing this in Parkinson’s — you need to know which proteins mark synapses that are more vulnerable to loss.

You’ve mentioned tracking protein movements. Why is that important?
Understanding how synaptic proteins move is likely to be fundamental to understanding synaptic function. Proteins can leave a synapse, enter a synapse, change their position within a synapse, engage with different partners within a given synapse. No protein only binds to the one or two molecules that we draw in our diagram — it may interact with a multitude of other partners.
To track membrane proteins, we need antibodies or nanobodies that recognize the extracellular domains — the smaller the better, so they can penetrate the synaptic cleft.
You say a protein may interact with many more partners than your diagrams show. Do researchers need to be looking to extend the list?
Yes, I think we are now in a position where we can be more systematic and really develop catalogs of synaptic proteins.
There are now proteomic approaches called proximity labeling that allow us to map the protein composition of synapses in an unbiased way.
How do those methods work?
Proximity labeling works by labeling proteins that are adjacent to reporter proteins so you can purify them. You can target reporters to distinct types of neurons in genetically controlled ways, using mouse models that express recombinases in those particular neurons. And then you can use the biochemical properties of the reporters to target them to distinct compartments of synapses. So it’s an intersectional approach.
The mouse models are established, and reporters have been developed. Now the challenge will be to put people together, and funding, to do this in as systematic a way as possible.
Of course, that will create more targets for which we need antibodies!
Wow. Well, right now, what antibody tools feel most needed?
I would like a panel of antibodies against bona fide synapse-organizing proteins that allow me to detect those proteins side-by-side — and compare how they affect each other’s expression, how they work together to instruct synaptic properties, how they create specific connections.
That has been a major impediment for the field: getting good, specific antibodies — and antibodies that can be generated on a scale that they can become universal tools, used across labs.
It’s interesting that you bring up resource sharing as a top priority need.
Ah, the field needs antibodies that can be used equally by investigators who have a lot of experience but also by people who come new to the field.
We need shared resources that have been validated and well-characterized, and that are accessible to anyone who wants to enter the field, rather than people having to just order something out of a catalog that’s advertised to recognize the protein they care about. Having a more standardized set of tools that allows for comparison of results across labs is important.
Source: Thomas Biederer, thomas.biederer@yale.edu
Writer:
Megan Talkington, megan.talkington@proteininnovation.org
Caitlin Faulds and Trisha Gura contributed to this story.
About IPI
The Institute for Protein Innovation is pioneering a new approach to scientific discovery and collaboration. As a nonprofit research institute, we provide the biomedical research community with synthetic antibodies and deep protein expertise, empowering scientists to explore fundamental biological processes and pinpoint new targets for therapeutic development. Our mission is to advance protein science to accelerate research and improve human health. For more information, visit proteininnovation.org or follow us on social media, @ipiproteins.

