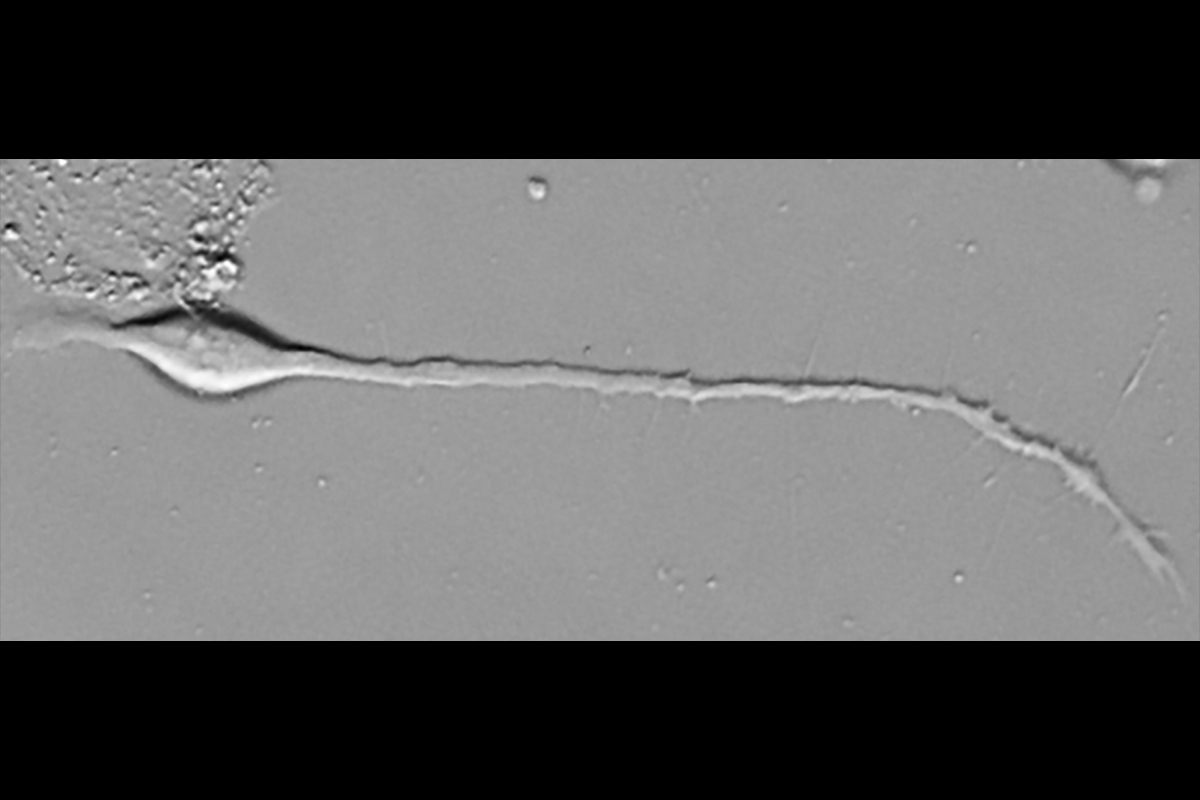
Early in development, an embryo begins an epic undertaking: the building of its nervous system. As part of the process, neurons send out axons on extraordinary journeys to distant targets. The axons find their way guided by constellations of extracellular signaling molecules — some attractive, others repulsive — that interact with cell surface receptors on the axon tip, the growth cone. Charting these crucial receptor-ligand pairs, and exploring their complex signaling mechanisms, captivates Alex Jaworski, an associate professor of brain science at Brown University.
In a recent seminar hosted by the Institute for Protein Innovation (IPI), Jaworski presented findings from his lab about several of the more unconventional of these receptors operating at the axon growth cone: Robo3, Punc, Nope and Protogenin, and their ligands NELL2 and WFIKKN2.
We spoke with Jaworski about what motivates his investigation of axon guidance machinery, and which big questions the field is now set to tackle. Our interview has been edited for length and clarity.
What makes nervous system wiring such an interesting problem to study?
The sheer complexity — the number and the pattern of connections. How is it possible that this really complex information processing system is built all by itself when you’re an embryo, based on a bunch of fairly simple molecular rules? To me, that’s a fascinating question.

Looking across evolution, it’s interesting that there are both really conserved features that have carried through from the common ancestor of fly and man, and there are other things that happened much more recently. Is something that changed in these molecules over the course of evolution what makes the nervous system look different? Or do you just add additional molecules to the toolkit, or combine these things in different ways, and that’s how a new wiring pattern emerges?
IPI is developing synthetic antibodies for axon guidance molecules. Why are new protein-detecting reagents like these useful?
Antibodies are important tools to determine which cells express proteins of interest and where these proteins are localized within these cells, providing important clues about their function. My group’s work is a good example. We have shown that Punc, Nope and Protogenin are expressed in two populations of neurons, using RNA data — in situ hybridization and single-cell RNA sequencing. We’re proposing a model that says these receptors sit on the surface of the axon growth cones, and that’s where they interact with ligands. But we haven’t shown that the protein is actually expressed by these neurons, on their axons, on their growth cones, right? So this is all inferred.
Would it be fair to say that, while there’s always a gap between mRNA and protein expression, there is an even wider gulf for proteins that operate at the tip of an axon, far from where their mRNAs are synthesized in the nucleus?
Absolutely. There’s a gap between mRNA and protein, because maybe the mRNA is silent and doesn’t even get translated. But on top of that, even if the protein is made, is it actually where we think it’s acting, in the growth cone?
Seeing your protein — it’s really important.
You’ve been studying axon guidance for much of your career. Over the years, has there been something that’s surprised you?
I’m still kind of surprised by how much we just don’t know.
Labs have done an amazing job over the years of elaborating the molecular toolkit for axon guidance — identifying the wiring molecules, categorizing them as attractive or repulsive, figuring out how they signal. I think the next frontier is: How is this all coordinated and dynamically regulated at a global level?
The growing axon of a mouse dorsal root ganglion sensory neuron in vitro turns away from the extracellular signaling molecule WFIKKN2. Video courtesy of Alex Jaworski.
Can you say more about that?
Let’s assume there are 150 different axon guidance receptors. How does a neuron decide, at this particular time, I’m going to express seven of those, and then a little bit later I’m going to express three of the same but another five additional ones? We don’t understand this at all.
Further, let’s say you have five different guidance receptors on a growth cone, and they all are engaged with their ligands. Some of them are going to tell the neuron it should be attracted; some of them tell it to be repelled. Somehow there needs to be crosstalk between these signaling pathways. How that works, and how the neuron ultimately synthesizes all this information into a coherent response, we also don’t understand.
What sorts of experiments do you think need to happen to start answering those questions?
The development of single-cell RNA sequencing has helped the field already, because we have a good way now to look at gene expression in individual neurons at specific time points. For example, this is how my lab discovered that there probably is a change in gene expression that drives commissural neurons to switch their guidance behavior as they cross the nervous system midline. And people have started mining these datasets and can map out gene expression batteries that are active during different stages of axon growth — entire groups of genes that are turned on at specific times to do certain things.
But how are these transcriptomic changes triggered? How is the expression of these genes controlled? Are they co-regulated, or are they regulated piecemeal? There are a lot of questions to be asked about the regulation of gene expression.
And then the question about integration and crosstalk in the growth cone. That, I think, requires some hardcore biochemistry and super-resolution imaging to look at local events in the growth cone.
Could antibodies be helpful for studying that crosstalk in the growth cone?
Yes. Let’s say you know that your neuron of interest expresses Robo3 and Robo2 at the same time. We know there’s supposed to be inhibition — Robo3 somehow shuts down Robo2’s signaling pathway — but we don’t even know if they’re in the same location. If you had excellent antibodies, you could perform super-resolution imaging on individual growth cones, and you might see that these receptors are concentrated in the same regions. Or maybe they’re spatially segregated, which would immediately exclude a physical interaction. So I think having good antibodies is going to be really important.
Source:
Alex Jaworski, alexander_jaworski@brown.edu
Writer:
Megan Talkington, megan.talkington@proteininnovation.org
Caitlin Faulds and Trisha Gura contributed to this story.
About IPI
The Institute for Protein Innovation is pioneering a new approach to scientific discovery and collaboration. As a nonprofit research institute, we provide the biomedical research community with synthetic antibodies and deep protein expertise, empowering scientists to explore fundamental biological processes and pinpoint new targets for therapeutic development. Our mission is to advance protein science to accelerate research and improve human health. For more information, visit proteininnovation.org or follow us on social media, @ipiproteins.