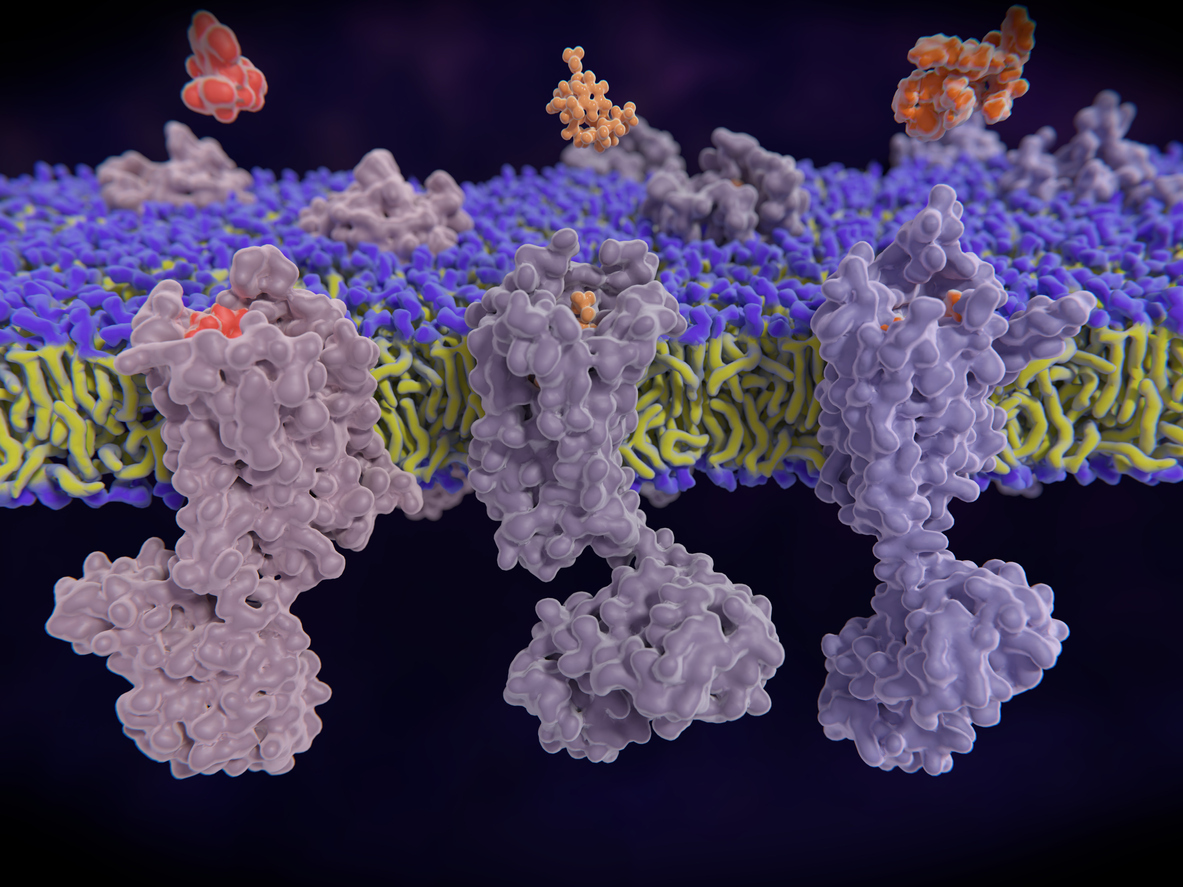
An opioid crisis in the US is not news. Indeed, the Centers for Disease Control and Prevention estimate nearly 841,000 people in the US have died in the last two decades from a drug overdose, and more than 70% of those deaths involved an opioid.
Opioids are powerful painkillers. They work through the nervous system or via specific receptors in the brain to reduce the intensity of pain. The problem with current opioids—and part of the reason the death rate from their overdose has increased more than six times in the last two decades—is specificity. People feel pain because their tissue has been damaged by trauma or surgery. But painkillers, like the synthetic opioid fentanyl, affect both local tissue and the entire body and brain, creating sedative effects that are addictive.
“Fentanyl basically makes you high,” says Rob Meijers, Head of the Antibody Platform at the Institute for Protein Innovation. “It would be nice if instead, painkillers would address only the areas of the trauma.”
To unearth such a discriminating narcotic, Meijers has joined forces with physician-scientist Christoph Stein at Charité – Universitätsmedizin and computational scientist Marcus Weber at Zuse Institute, both in Berlin, Germany. The team has been awarded a grant by the National Science Foundation (NSF) to deploy computational science and synthetic antibodies to better understand the opioid-receptor, pain-signaling pathway. Their results could lead to a new class of pain relievers that address trauma without addictive side effects.
Simulating the Shape of Pain
The story of this project begins with an old idea. When tissue is inflamed or traumatized, its pH drops. That’s because cells are disrupted, spilling out their acidic contents. Long ago, Stein surmised that opioid receptors respond to this pH drop by changing their shape. If one could find a painkiller that specifically targets the opioid receptor in its pain-signaling conformation, he thought, it might spare the rest of the brain and body.
But a controversy arose as to whether this elusive shapeshifter even exists.
To try and solve it, Weber came up with the idea of deploying traditional molecular dynamics (MD) simulations and powerful artificial intelligence (AI) to mimic on a computer the shifting conformations of opioid receptors.
The task is not easy. Opioid receptors are members of a large, scientifically infamous family called “G-protein coupled receptors” (GPCRs). They reside on the surface of cells and work by translating signals coming from outside the cells (e.g. binding of drugs) into the activation of processes inside of cells. The signaling occurs through three-dimensional shape changes, which many factors can influence, including the binding of the receptor to its partner (called a ligand) or the pH of the external environment.
Weber is deploying a powerful approach that models the receptor’s intricate molecular structure and calculates the energy dynamics between atoms and molecules within it, both alone and when exposed to its ligands or changing environment. Because the molecular interactions are in constant flux, the work is computationally expensive; researchers can only model microseconds of receptor shifting, even when using supercomputers. One needs seconds-long representations to even begin to pinpoint the right shape.
Weber developed an innovative approach in which he captures short bursts, analogous to Tik Tok snippets, and uses AI to string them together into longer videos of receptor shapeshifting. Once he can simulate the architecture of the native opioid receptor, he will try to mimic its pathological state by virtually lowering pH of the modeling system or adding free radicals. His objective is to yield the best 20 representations of a conformation that predict pain signaling.
Making It at the Bench
While Weber works his computational magic, Meijers’ team, led by principal scientist Shaotong Zhu, is conducting its own feats in the laboratory. Zhu is first creating the actual opioid receptor, using molecular and structural biology techniques. Her task is non-trivial, given the opioid receptor resides in a membrane and therefore needs mammalian cells and a lot of expertise to produce it in pure form.
Once the receptor is fashioned, the IPI team will then use its yeast display technology to discover synthetic antibodies that recognize different regions of the receptor.
“These will be the first recombinant antibodies for human opioid receptors,” says Meijers, “diagnostic tools to identify neurons that have opioid receptors on their surface.”
Such antibodies are currently lacking, in part, because the receptors, like many neurological proteins, are nearly identical in mice and humans. That means traditional antibody-development approaches that involve animals, like mouse hybridoma technologies, have severe limitations.
Because the IPI team uses yeast to generate its antibodies, it can get around the animal constraint. In addition, yeast can withstand changing environmental conditions like low pH or the addition of hydrogen peroxide (to simulate the nasty free radicals generated by tissue damage). Thus, the IPI team can place its opioid receptors into the real conditions that trigger the potential pain-signaling shape change and use that to generate a second or third panel of antibodies that bind only to the elusive pathological state.
“We’ll have a state for the (opioid) binding, a state for the normal receptor, and a state for the pathological receptor,” explains Meijers.
Putting It All Together
As the biochemical work unfolds, Weber will continually feed the IPI team clues from his computer simulations, such as mutations that might be made in the receptor that better enable a desired state. His input can help the team achieve a fait accompli, finding antibodies that not only recognize the opioid receptor in its pathological state but actually “lock” it into that conformation so that researchers can better study it.
Those studies are the trademark of the third collaborator on the project: Stein. IPI will send him the antibodies it develops, and the Charité lab will test the binders in neurological studies, identifying which neurons have which state of opioid receptors on their surface and the effects of opioids on those cells under conditions of trauma.
While computation was the top interest of the NSF in sponsoring the grant, followed by the biochemistry of opioid signaling, still there was a third objective. The project is meant to facilitate cross-cultural collaboration. In fact, IPI in Boston will be sending a postdoc to Berlin, and vice versa.
“In Berlin, there is a physician-scientist and a computational biologist,” says Meijers, “In Boston, we have GPCR experts and protein scientists, who can make antibodies specifically for the opioid receptors. It’s a perfect collaboration.”