For the body to function, cells must decide. Embryonic cells elect to reproduce and shape into budding limbs. Immune cells choose to leave the bloodstream en route to an infection site. Cancer cells ignore precedent and divide uncontrollably without regard.
When and where to adhere, shape-shift or migrate is a cellular judgment, “a life-or-death decision,” says biophysicist Taekjip “TJ” Ha at Johns Hopkins University School of Medicine in Baltimore, Maryland. “Because cells are in contact with the environment through receptors … the cell has to somehow make and integrate thousands of single-molecule, mechanical measurements before making a decision.”
Just how cells determine their fate was explored in a recent Nature Communications article by Ha, alongside his lab group and researchers at Boston Children’s Hospital, the National Institutes of Health and the Institute for Protein Innovation (IPI). The team reported novel insights into the intricate mechanics of a group of transmembrane receptors, dubbed integrins, that sense, integrate and convert mechanical stimuli into biochemical signals that steer intracellular change.
The results show how a relatively small number of integrins harness chemical cues and respond to physical prompting orchestrated by force, tension and rigidity. Though basic in nature, the work sheds light on the pathological mechanisms at play in certain autoimmune conditions and cancers — and may ultimately inform therapeutic development.
The question
Since the discovery of the first integrins in the mid-1980s, knowledge of this family of membrane receptors has exploded. Launched by a trio of early researchers — IPI co-founder Timothy Springer of Harvard University, Richard Hynes of the Massachusetts Institute for Technology and Erkki Ruoslahti of the Sanford Burnham Prebys Medical Discovery Institute — scientists have established integrins as transmembrane bridges, sending signals between extracellular scaffolding and the intracellular cytoskeleton.
Investigators have uncovered a 24-member strong family tree and shown each integrin to be composed of one of 18 alpha and one of eight beta subunits. Scientists have also confirmed integrins’ role as mechanotransducers that respond to and transmit force both outside in and inside out.
But the quantification of those forces and the mechanics of their outcomes have remained obscure — that is where this story begins.
The tools
Ha was trained as a physicist, but shifted to biomedical engineering after realizing his skillset was “more useful for biology than physics.” He built a lab renowned for its ability to gauge the activity of biomolecules using fluorescence and laser-driven instruments called optical tweezers.
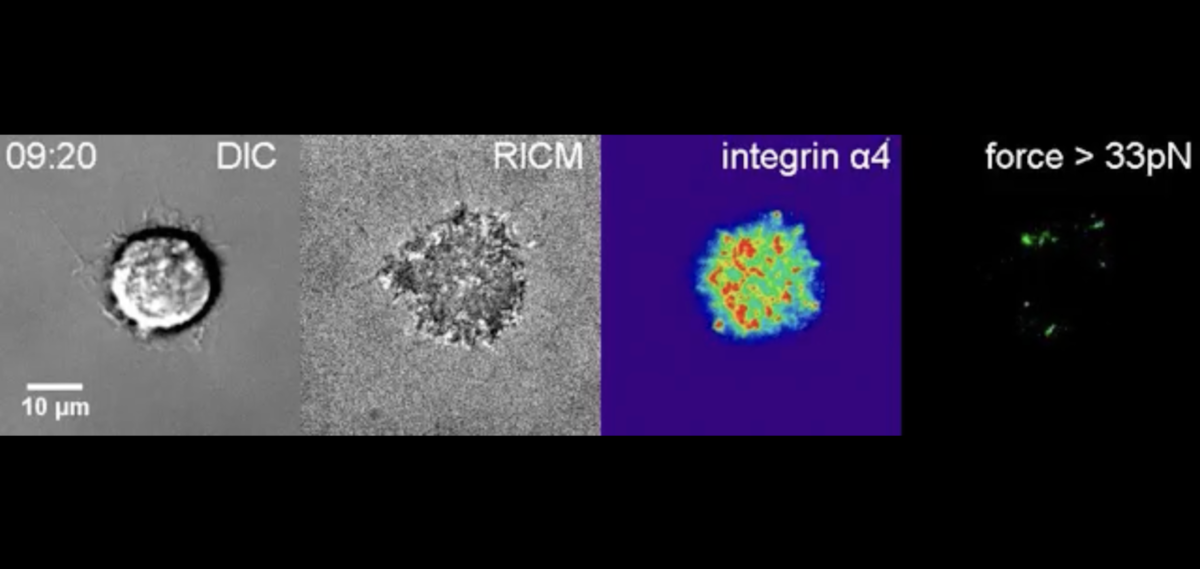
In 2013, his postdoc Xuefeng Wang, now at Iowa State University, invented a nanoscopic tool, a DNA-based tension gauge tether (TGT), to measure the force applied to the bond between a single receptor and its binding partner or ligand.
To construct the tether, Wang exploited the inherent potency of DNA’s signature double-stranded helix. He linked one strand to a molecular biotin tag and anchored this complex to a surface. He hitched the other to a ligand of choice. When a force pulled on the ligand, the tension on the tether would increase to breaking point, eventually rupturing the DNA’s dual strands and igniting a fluorescent probe.
By deploying a range of tethers, with tunable tension tolerances from 12 to 54 piconewtons, the researchers could make real-time measurements of the force required to activate a single molecule in live cells. This was an invaluable step in spotlighting the biophysical mechanisms of integrins.
Wang and Ha next tailored a set of TGTs to target specific integrin family members, tacking on three amino acids — arginine, glycine and aspartic acid (RGD) — representing a small binding motif. Using the RGD-TGTs, the researchers found the force necessary to prod a single integrin to signal cell spreading was relatively large, 40 piconewtons.
This was the first time scientists had quantified a single integrin’s biophysical trigger. The results were published in Science.
The cells
That same year, Ha gave a public lecture at a ski meeting in Aspen. Springer, who had been exploring α4β1, the first of another line of integrins, was in the crowd. Upon hearing the talk, Springer stood up alone and gave Ha a standing ovation.
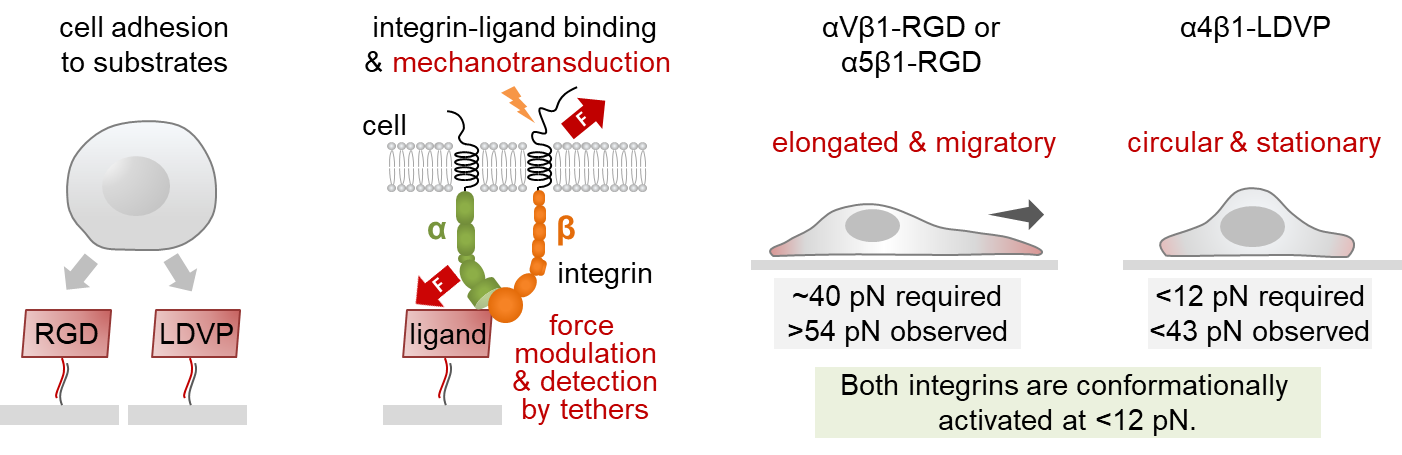
Though related to RGD-binding integrins, integrin α4β1 bound ligands with a different amino acid motif: leucine, valine, aspartic acid and proline (LVDP). Springer knew LVDP- and RGD-binding integrins were distinct in how they behaved and prompted cells to stick to a substrate and flatten out, a key precursor to cell movement. Now, Ha had a method to determine exactly how much force it took to initiate cell spreading.
“I said to TJ, ‘Why don’t we make a tension gauge tether with the α4β1 ligand and compare it to your RGD tethers,’” Springer recalls. “‘I think that there will be differences between them.’”
And so, they collaborated. Springer’s lab provided an LVDP-bearing ligand. With it, Ha’s postdoc Myung Hyun Jo created a handful of new TGTs to track adhesion, spreading and migration of human foreskin cells, which express both integrin types.
Jo showed RGD-binding integrins demanded higher tension before they would push cells to spread. Once secured to a surface, the cells morphed asymmetrically, elongating and migrating — reenacting a scenario like wound healing. By contrast, LVDP-binding integrins required and conferred less force, encouraging the cells to hunker down into symmetrical, immobile circles.
The results suggest that not only were there two systems in play, triggering dramatically different cellular responses, but also, “cellular function and morphology are not predetermined,” Jo says. “They depend on the environment.”
The antibodies
To figure out which integrins were responding to what environmental forces to cue differences in cell spreading, the researchers needed a way to discern the actions of the culprit receptors.
This feat proved extremely challenging due to the tight-knit integrin family. Each member shares key protein regions with the other receptors, making their individual roles difficult to distinguish. Because foreskin fibroblasts express multiple RGD-binding integrins, inhibiting one receptor at a time often fails because another steps in to muddy results.
But scientists at IPI had a solution, stemming from an antibody-generating technology known as yeast display which relies on a library of one billion yeast cells, each engineered to express a binding fragment called a Fab. Using the technology, postdoc Jing Li in the Springer lab and the IPI team discovered synthetic Fabs that bound specifically to a unique amino acid region of a subset of the RGD-binding integrins with the αV subunit. Meanwhile, the Springer lab rounded out the RGD-binding integrin toolbox by producing mAb16, an antibody from a hybridoma directed against another RGD-binding integrin, α5β1.
Using these antibodies, the investigators inhibited the entire integrin cast, both individually and in combination. To everyone’s surprise, the work showed for the first time that an unostentatious integrin, αVβ1, was the leading actor initiating cell spreading. Two other RGD-binding integrins, αVβ3 and αVβ5, played supporting roles in the early stages of adhesion.
The team also showed that the mechanical tension threshold required for cell spreading was higher than that required for integrin activation, suggesting that cell spreading was not incited by integrin activation directly, but instead through a chain reaction. Smaller forces switched on the integrins, spurring assembly of the cytoskeleton and pulling on the integrin, thus generating tension strong enough to drive the cells to adhere and spread.

The implications
While fundamental, the work also has clinical implications. Faulty expression and signaling in some RGD-binding integrins are associated with increased tumor progression and decreased patient survival. Antibodies and small molecules that specifically block these receptors have entered late-stage clinical trials.
But key to these therapeutic advances is basic science that dissects the activity of each integrin subtype and finds precise methods to knock out confounding variables.
“Figuring out how these molecules work is amazing,” Li says. “Not only the structure of integrins but the energy landscape, how the different conformations bind ligands differently, screening tumors to see which integrin can potentially work as a biomarker for cancer targeting. I think it’s all fantastic.”
The Institute for Protein Innovation is pioneering a new approach to scientific discovery and collaboration. As a nonprofit research institute, we provide the biomedical research community with synthetic antibodies and deep protein expertise, empowering scientists to explore fundamental biological processes and pinpoint new targets for therapeutic development. Our purpose is to advance protein science to accelerate research and improve human health. For more information, visit https://proteininnovation.org/ or follow us on social media, @ipiproteins.
Sources: Myung Hyun Jo, mjo5@jhu.edu; Taekjip Ha, tjha@jhu.edu; Jing Li and Timothy Springer, springer_lab@crystal.harvard.edu
Author: Trisha Gura, trisha.gura@proteininnovation.org