Antibody purification is often taken for granted. It’s an essential step in isolating desirable antibodies for research and drug development and a standard part of antibody production at the Institute for Protein Innovation (IPI) — so standard some view it as the “most tedious part,” according to IPI scientist Roushu Zhang.
“Basically nothing should be expected to go wrong,” said Zhang, who has led antibody production at IPI for two years.
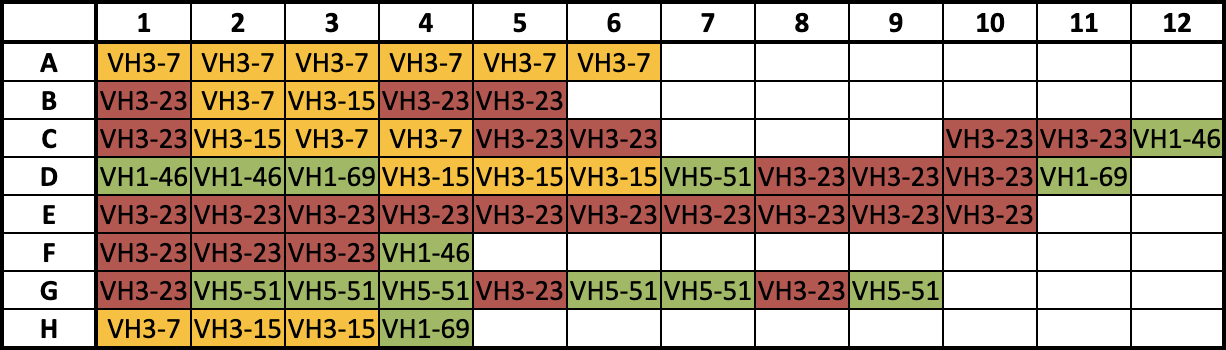
But, occasionally, surprises come up — like when Zhang and the IPI Antibody Production team attempted to purify VH3-encoded antibodies, a class of antibodies with a particular genetic makeup on the target binding frame of their heavy chains.
“That’s from plate 85 — I still remember the number,” Zhang said.
Zhang typically sees around a 99% success rate for antibody purification. But when her team ran plate 85 in early 2022, the VH3-23 antibodies in more than half of the wells failed to separate out. She repeated the experiment again and again only to find the same result — zero recovery for half the plate.
Dubbing the plate “specifically cursed,” Zhang set out to find out what was happening within the experimental processes.
Finding the problem
After dismissing experimental anomalies or one-off lab errors as the issue, Zhang dug deep into technical terrain.
Antibody purification can be done in a number of ways, but it’s commonly performed via Protein A affinity chromatography. The technique uses Protein A (ProA), a protein derived from a naturally occurring bacteria strain and commonly conjugated to a solid support to create a resin included in elution kits, to grasp an antibody and hold it on a magnetic column. This allows the rest of the initial solution to flow out in repeated washes. The antibody is then eluted, allowing that resin-antibody attachment to loosen and leave only the isolated antibody behind.
When purification fails, often the response is to make the elution conditions more acidic, harsh enough to strip the resin from the proteins no matter how tightly they’re bound. So Zhang raised the acidity of the buffer solution from pH 3 to pH 2. The team found they could isolate the antibodies — but at a cost: destabilization.
Harsh conditions caused the antibody to unravel and unfold. This degradation exposed new areas of the VH3 antibodies, making them sticky and prone to cross-reaction.
“We don’t want to see the antibody quality dropping that significantly,” she said.
She needed a different way to purify VH3 antibodies — without the side effects.

A VH3 solution
Generally, ProA latches onto an antibody by anchoring its crystallizable fragment (Fc), leaving the two-armed antigen-binding fragment (Fab) untouched. But Zhang suspected there might be a particular issue in how the resin interacted with VH3 antibodies.
After an extensive literature review, Zhang found other labs had experienced the same issue. The resin was overzealous in interacting with VH3, clinging to both the tail and binding arms of the antibodies in a so-called “bivalent effect,” Zhang said. The result was a bond too strong to be broken by standard elution.
She’d found the root of the plate 85 problem — and it was clear they needed another way to solve it.
Engineering Protein A to Z
Zhang was up to the task. She joined IPI in March 2021 after studying HIV-1 and SARS-CoV2 viral vaccine design and glycosylation during her Ph.D. at the University of Maryland. She’s driven by the challenge of using chemistry to piece together biological processes. And she saw VH3 purification as a new puzzle to be solved.
She knew ProA had five key homologous binding domains: E, D, A, B and C. These five domains were relatively similar in structure and all were culprits in cross-reaction with the VH3 Fab region. But the B domain could be exploited and mutated to create a better performing VH3-indifferent binding partner — so she gave it a shot.
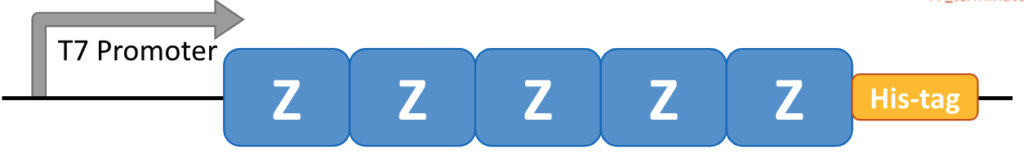
First, the B domain of ProA was mutated at A1V and G29A to morph it into a new, structurally similar Z domain that would interact only with the Fc region. Zhang cloned the Z domain five times and fused the copies to form a recombinant pentameter that replicated ProA’s native folding structure.
She dubbed this new resin “protein Z” (ProZ), produced it in E. coli and conjugated it to magnetic beads. Then, without increasing the buffer’s acidity, she mixed it with VH3 for high-throughput purification. It worked.
The purification rate for the new VH3 plates jumped from approximately one-half to near 100% — with no drop in antibody quality and stability.
ProZ may have other applications, but it has specific utility in addressing VH3 purification issues, effectively filling a gap ProA could not. And though the lab doesn’t often work with the troublesome antibody family, it’s important to have the solution at the ready.
“We want to have a technology that is available,” Zhang said. “If anybody is interested in ProZ, they’re welcome to try it.”
The Institute for Protein Innovation is pioneering a new approach to scientific discovery and collaboration. As a nonprofit research institute, we provide the biomedical research community with synthetic antibodies and deep protein expertise, empowering scientists to explore fundamental biological processes and pinpoint new targets for therapeutic development. Our mission is to advance protein science to accelerate research and improve human health.
For more information, visit proteininnovation.org or follow us on social media, @ipiproteins.
To learn more about ProZ, contact us at info@proteininnovation.org.
Source: Roushu Zhang, roushu.zhang@proteininnovation.org
Author: Caitlin Faulds, caitlin.faulds@proteininnovation.org