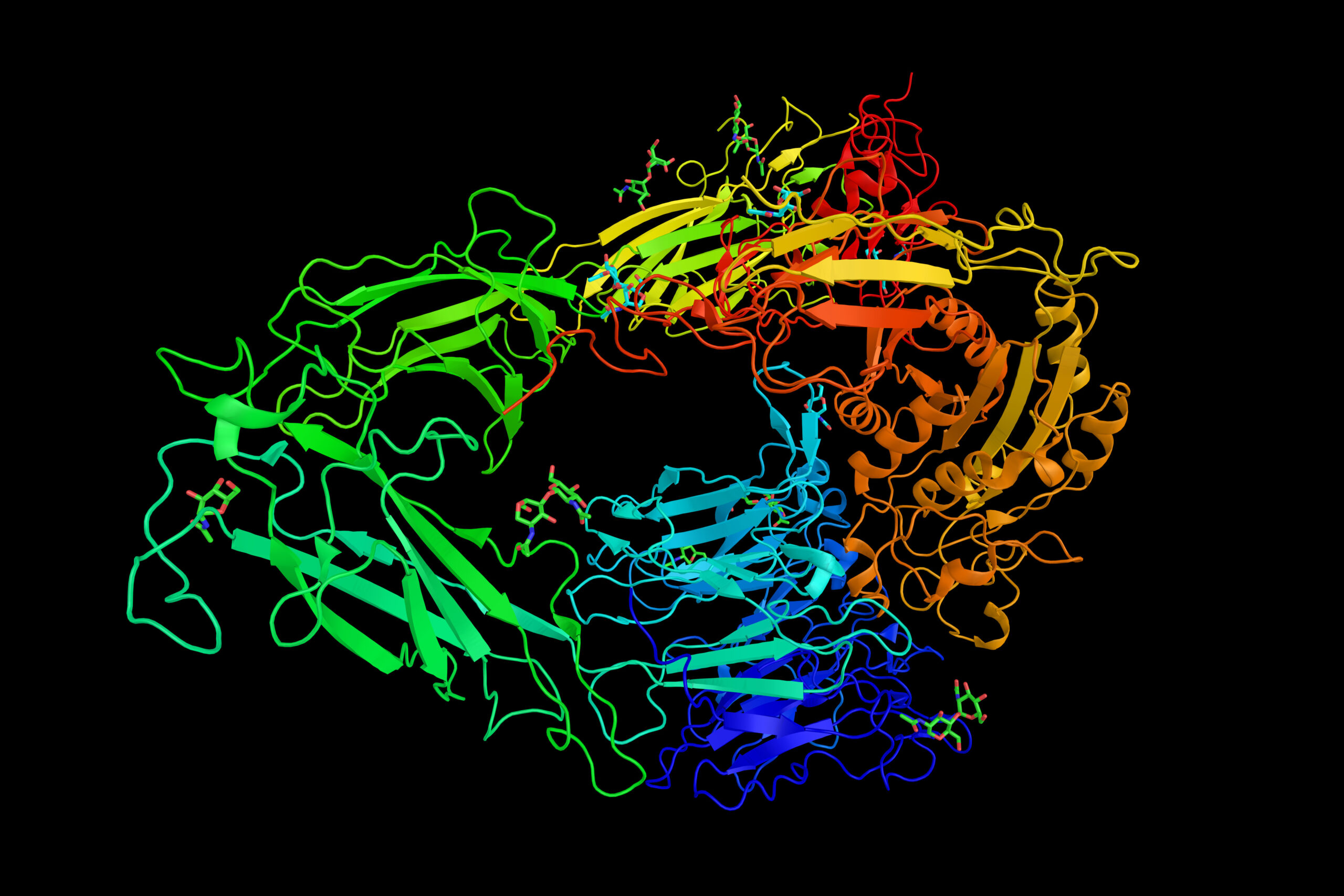
More than 600 million years ago, out of the depths of the metazoa, a protein family emerged — one that would persist through evolution and come to shape life today.
Now known as integrins, these aptly named transmembrane signaling proteins play a pivotal role, connecting cells to their surroundings and controlling a variety of biological processes, from cell growth and migration to tissue repair and immune response.
And they keep the act up across species. They’ve been detected in multicellular organisms ranging from humans to mice to the sponge — a relic of ancient life and “sister” to all other animals.
But what exactly is this highly conserved, highly elusive protein family? And how can better understanding their biology drive therapeutic discovery?
What’s an integrin?
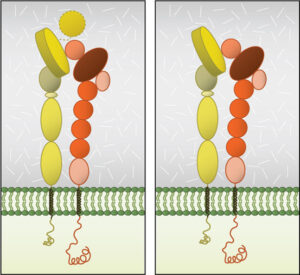
The key here is in the name.
Integrins are “integral” transmembrane protein complexes found on nearly every cell in the human body — all except mature erythrocytes, or red blood cells.
They work as essential chemical and mechanical middlemen — “integrating” the cellular cytoskeleton to the surrounding soup of secreted polysaccharides and proteins called the extracellular matrix (ECM).
The name — proposed by early integrin researchers in 1986 — further denotes their role in ensuring the “integrity” and structural assembly of the cytoskeleton and the ECM.
Integral, integrating, integrity — integrins. Simple.
But there’s a little more to them than that.
Getting into the details
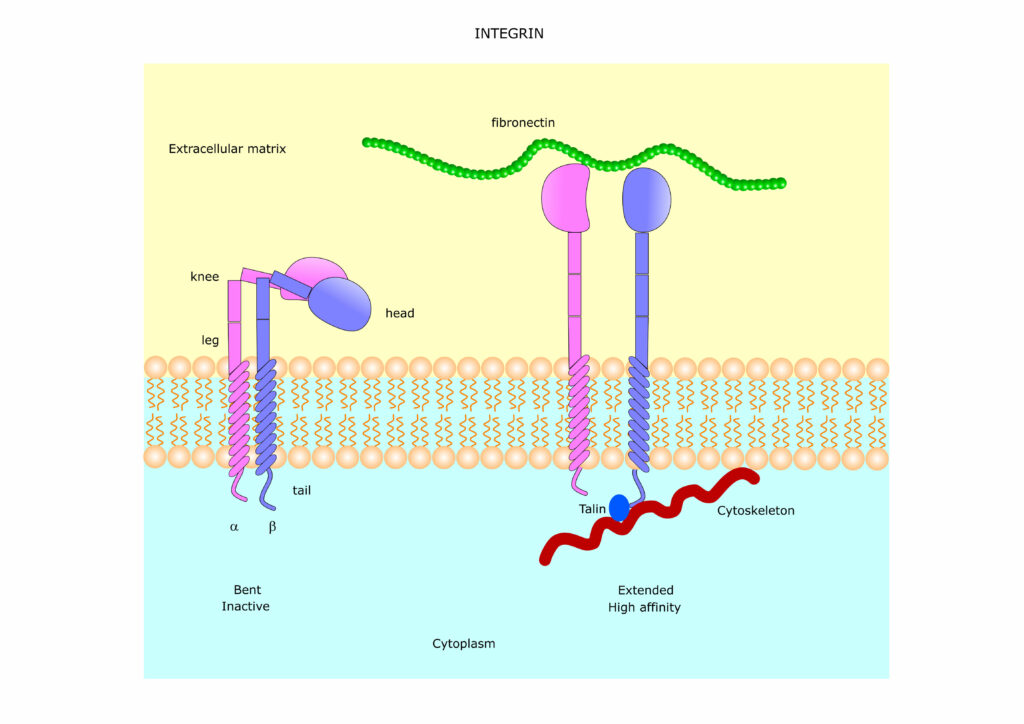
There are 24 known mammalian integrins, all heterodimers composed of one of 18 alpha and one of eight beta subunits. Both subunits have short intracellular ends and large extracellular heads that bridge the cell membrane, physically connecting the cytoskeleton and the ECM.
This positioning gives the receptor unique bidirectional abilities, allowing cellular cues to be relayed both “outside in” and “inside out.”
Outside the cell, integrins are promiscuous adhesion receptors. They bind to a wide range of cell membrane proteins and extracellular glycoproteins, including fibronectins, laminins and collagens — depending on their subunit setup.
But exactly when and how strongly the integrin attaches depends on whether or not they’re activated. In their resting state, integrins exist in a low-affinity mode with a bent extracellular end. But this end can be activated through interaction with ligands outside the cell — such as ICAMs — or proteins inside the cell — namely talin. This biochemical stimulus, no matter where it occurs, cues outside conformational change. The extracellular end opens and elongates, morphing into a high-affinity state to better cement ligands to the membrane.
“It’s a pretty complicated connection that does different things,” said Richard Hynes, an early integrin discoverer and professor in cancer research at the Massachusetts Institute for Technology.
When firmly adhered to the membrane, ligands can trigger internal signal cascades that change cell polarity, brace cytoskeletal structure and encourage cell survival and proliferation.
A stronger bind also means integrins apply more force to their targets. That force helps mold the matrix into patterns, which impact cell migration and can play a role in cancer spread and invasion.
“Integrins are what hold us together,” Hynes said. “When you knock them out, something goes wrong.”
So how’d they figure all this out?
In the mid-1980s, Hynes, Erkki Ruoslahti and Timothy Springer were all working different angles of the same problem — though they didn’t know it yet.
Hynes, then a founding faculty member of the MIT Center for Cancer Research, and Ruoslahti, of the Sanford Burnham Prebys Medical Discovery Institute in San Diego, were about a decade deep into independent research on fibronectin — a protein abundant on the outside of normal cells and missing from cancer cells.
Hynes and MIT colleague Antonia Destree had worked out that extracellular fibronectin was associated with intracellular actin — a finding that was confirmed by others and suggested an undefined receptor was serving as a crucial transmembrane bridge.
Meanwhile, the Ruoslahti lab had successfully sequenced a small cell-binding domain of fibronectin, drilling down to a critical three-amino acid stretch — arginylglycylaspartic acid (RGD) — that mediated integrin attachment. Using this sequence, Ruoslahti led the search for additional RGD-containing proteins, finding vitronectin and its receptor.
These disparate lines of inquiry led both labs to a previously known platelet membrane receptor, glycoprotein IIb/IIIa (gpIIb/IIIa). On opposite sides of the country, they realized that gpIIb/IIIa, a fibrinogen receptor responsible for blood clotting and deficient in the bleeding disorder Glanzmann thrombasthenia, bound to fibronectin — and was also related to the vitronectin receptor.


The Hynes lab had gone on to sequence the beta subunit of an avian matrix receptor — thought to be homologous with the gpIIb/IIIa complex — when a serendipitous inquiry into the relationship between gpIIb/IIIa and two similarly heterodimeric receptors led them to Springer.
“I discovered he was across the river,” Hynes said of Springer, then an early professor at Harvard Medical School and the Dana Farber Cancer Institute. “So I called him up and said, ‘Can we get together and talk?’”
Springer, now co-founder of the Institute for Protein Innovation (IPI), had spent years employing monoclonal antibodies to discern cell surface molecules required for cell to cell recognition in the immune system. So far, he had found three similar receptors — lymphocyte function-associated antigen 1 (LFA-1), macrophage-1 antigen (Mac-1) and p150,95.
By the mid-1980s, Springer was sure of the receptors’ role in adhesion and signaling and was investigating their link to hereditary leukocyte adhesion deficiency and autoimmune diseases. He was midway through sequencing their beta portions when Hynes came calling.
Together, Hynes, Springer and their colleagues combed through reams of computer printouts to compare protein sequences — spotting the receptors’ characteristic cysteine-rich pattern almost instantly.
“It took me 10 seconds to recognize that it was homologous,” Hynes said. “It was one of those eureka moments. It was fantastic.”
Excited by the monumental find, Hynes organized a Gordon Research Conference in early 1987 to map out the branches of this apparent family tree. He invited everyone he could identify — biologists and hematologists to immunologists and fruit fly experts — that he suspected may be working on related receptors. By the conference’s end, the integrin family had more than doubled.
“Sometimes some of the most profound problems aren’t easy to understand from one perspective alone,” Springer said. “And that’s the case for integrins.”
In 2022, the discovery garnered the trio the Albert Lasker Award for Basic Medical Research, a prestigious award honoring scientists whose fundamental discoveries have opened new areas of biomedical science.
What’s this mean for medicine?
That moment struck a match, initiating a “virtual explosion” of work on integrins, wrote Ruoslahti in The Journal for Clinical Investigation in 1991.
“Aside from their biological importance to fundamental cellular processes, the medical importance of the integrins is rapidly being realized as well,” Ruoslahti continued.
With potential to treat a range of conditions — from autoimmune disease to inflammation and endometriosis to cancers — therapeutic interest in integrins has remained consistent over time.
In 1992, Springer founded his first company, LeukoSite, later acquired by Millennium and then Takeda Pharmaceuticals, in the hopes of shuttling one of his monoclonal antibodies into therapeutic use.
The resulting biologic, vedolizumab (Entyvio ®), which targets the ɑ4β7 integrin to prevent T cell migration to the gut, gained Food and Drug Administration (FDA) approval for the treatment of ulcerative colitis and Crohn’s disease in 2014. It’s since become a blockbuster drug and a leading integrin therapy.
Two additional antibody therapeutics and three small molecule drugs are currently FDA-approved to treat conditions including multiple sclerosis, dry eye disease, acute coronary syndrome and thrombotic cardiovascular events. Between 2015 to 2020, there were more than 130 clinical trials of anti-integrin therapies — many of which build up on early insights into integrin structure and function.
But the complexity of the receptor and the lack of target validation tools have slowed progress.
“Developing tools to really dissect and better map out structure-function relationships of integrins is going to be essential to better understand the biology, which is crucial to effectively exploit integrins as therapeutic targets,” said Yamina Berchiche, membrane protein expert and commercial development manager at IPI.
And antibodies, like those produced at IPI, can serve as a powerful weapon in this fight.
“I think that’s going to be a growth industry: making lots of different antibodies to integrins …,” Hynes said. “Antibodies are a very powerful thing.”
The Institute for Protein Innovation is pioneering a new approach to scientific discovery and collaboration. As a nonprofit research institute, we provide the biomedical research community with synthetic antibodies and deep protein expertise, empowering scientists to explore fundamental biological processes and pinpoint new targets for therapeutic development. Our mission is to advance protein science to accelerate research and improve human health. For more information, visit https://proteininnovation.org/ or follow us on social media, @ipiproteins.
Read more about Timothy Springer’s contributions to this research here.
Resources:
- The emergence of integrins: a personal and historical perspective. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3493146/
- Integrins. https://dm5migu4zj3pb.cloudfront.net/manuscripts/114000/114957/JCI91114957.pdf
- Integrins: A family of cell surface receptors. https://www.sciencedirect.com/science/article/pii/0092867487902339
- Integrins as biomechanical sensors of the microenvironment. https://www.nature.com/articles/s41580-019-0134-2
- Integrin inside-out signalling and the immunological synapse. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3294052/
- Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. https://www.nature.com/articles/309030a0
- A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. https://pubmed.ncbi.nlm.nih.gov/2412224/
- Relationships between fibronectin (LETS protein) and actin. https://www.cell.com/cell/pdf/0092-8674(78)90272-6.pdf
- Are integrins still practicable targets for anti-cancer therapy? https://pubmed.ncbi.nlm.nih.gov/31336983/
- Emerging therapeutic opportunities for integrin inhibitors. https://www.nature.com/articles/s41573-021-00284-4
- Talin — the master of integrin adhesions. https://journals.biologists.com/jcs/article/130/15/2435/56321/Talin-the-master-of-integrin-adhesions
- Integrin ligands at a glance. https://journals.biologists.com/jcs/article/119/19/3901/29120/Integrin-ligands-at-a-glance
- Integrin cytoplasmic tail interactions. https://pubs.acs.org/doi/full/10.1021/bi401596q
- Integrins: An overview of structural and functional aspects. https://www.ncbi.nlm.nih.gov/books/NBK6259/
- Evolution of the Integrin α and β Protein Families. https://link.springer.com/article/10.1007/s002390010134
- Integrins. https://www.ncbi.nlm.nih.gov/books/NBK26867/#:~:text=Integrins%20are%20the%20principal%20receptors,of%20its%20integrins%20from%20within
- Improved modeling of compositional heterogeneity supports sponges as sister to all other animals. https://www.cell.com/current-biology/fulltext/S0960-9822(17)31453-7
- Millenium mergers bring pipeline. https://www.nature.com/articles/nbt1299_1151a
- Point man on protein science. https://invivo.pharmaintelligence.informa.com/IV124550/Point-Man-On-Protein-Science
- LeukoSite. https://invivo.pharmaintelligence.informa.com/IV000013/LeukoSite
- This billionaire has quietly driven Boston’s biotech industry for decades. https://www.statnews.com/2022/07/29/this-billionaire-has-quietly-driven-bostons-biotech-industry-for-decades/
Sources:
Timothy Springer, springer_lab@crystal.harvard.edu
Richard Hynes, rhynes-admin@mit.edu
Yamina Berchiche, yamina.berchiche@proteininnovation.org